QUESTIONS ABOUT USING IRB NOVELUTION
Projects in IRBNet will not be available after August 23, 2026. Please be certain to download all pertinent records and close projects that are no longer active.
IRBNet projects should transition to IRB Novelution only for the following actions:
- Amendments
- Continuing Reviews
- Progress Reports
Any new projects need to be created in IRB Novelution.
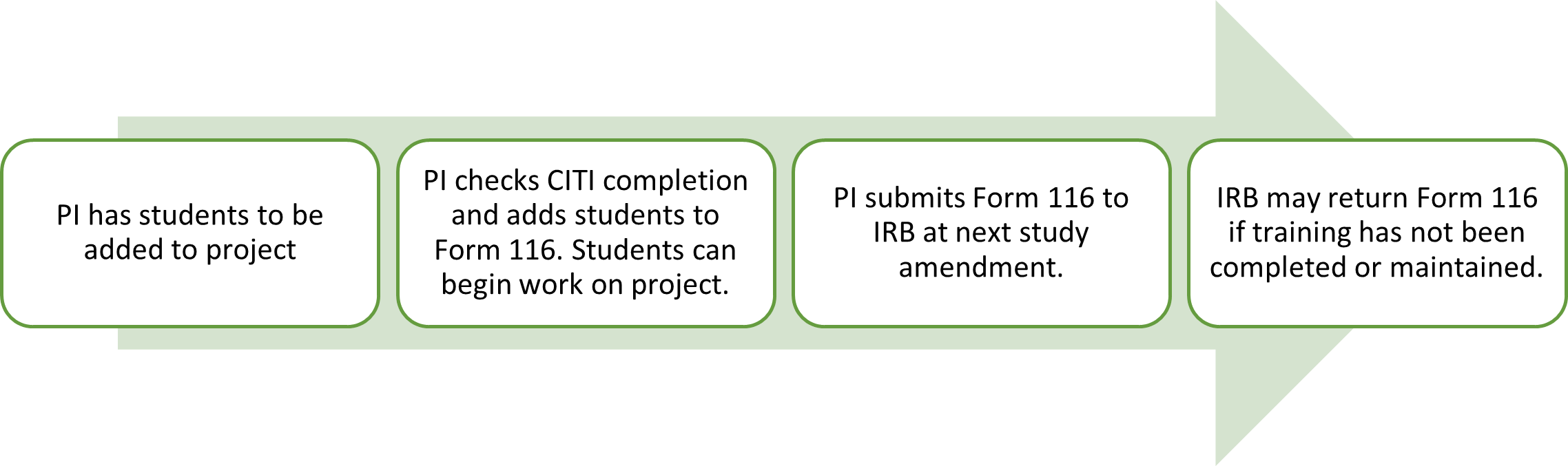
New Process for Study Personnel Updates
Starting October 1, 2024, minimal risk study changes in non-key study personnel do not need to be submitted via amendment. Instead, use Form 116, “Non-Key Study Personnel” to track changes and submit the form at the next study amendment. If there is a change in PI, Co-I, external collaborator, or other key personnel, these must still undergo an amendment review and approval prior to initiating the change. A sample process is illustrated below.
I submitted my application in IRB Novelution, what is the current status of my project?
IRB Novelution provides two places to check your project status. The first is at a bar up at the top which will give you the current status i.e. “Draft Submission pending”, “IRB Review pending” etc. The more detailed way to see the process timeline of your project is to visit the Requirements panel all the way at the bottom of your submission. There you can check that the appropriate pre-submission approvals such as PI certification, Departmental Approval and CITI training have been marked as “Completed”.
I was able to see my IRB Novelution project before but I cannot see it now, what is the issue?
If you use an @health.fau.edu email account, your login for Novelution is still your standard @fau.edu SSO login. You must use the @fau account to login, however any emails or notifications from the project will still be directed to your @health email.
The IRB tab is not showing up for me, how can I create a project?
If you are able to successfully to login to IRB Novelution it means your account is active and you should be able to view the IRB tab. Typically the issue is the browser magnification, click to Zoom out of the browser until the IRB tab is visible.
How do I transfer my projects from IRBNet into IRB Novelution?
IRBNet projects only need to be transitioned if you will be submitting an amendment, continuing review, or progress report. All other IRB related submissions, please contact researchintegrity@fau.edu before proceeding.
Create a new project in IRB Novelution and input the latest version of your IRBNet project details into the respective IRB Novelution field. Use the IRBNet to IRB Novelution Comparison Guide as a reference. You will need to go into each project in IRBNet and download all study related documents including but not limited to approval letters, consents, flyers and other recruitment materials, protocols, applications, and IRB request forms. These can also be attached into your IRB Novelution project for reference.
Do I have to create a new project in IRB Novelution?
Yes, all new submissions (new project, amendment, continuing review, etc) need to be submitted into the system to create a new record for them. Legacy documents from your IRBNet can be uploaded to your new projects to serve as reference. See below for specific project actions.
Can I still access my IRB documents in IRBNet?
Yes, IRBNet is not going away completely. You will still be able to log in to your account and will have access to your IRB documents but will not be able to submit anything through IRBNet. We strongly encourage you to make copies of all your records in IRBNet for your continued access, as IRBNet will close. Projects and their documents in IRBNet will only be avaialble until August 2026. Please be certain to transfer active projects to Novelution and close those that are no longer ongoing. Additionally, be certain to move your IRBNet documents including correspondence, protocols, and other study materials from IRBNet before it is unavailable. IRB staff will not be able to retrieve any records in IRBNet after August 2026.
I can’t link my CITI profile to IRB Novelution.
If you cannot link your CITI profile, the first step is to log in to your CITI account. Check that your Primacy Email is the same as your Florida Atlantic Workday account. If it is different, update the email and the systems will link within 24 hours. You may have to hit “Refresh” under the Requirements Panel in IRB Novelution to ensure they are linked.
I have an existing project that was approved in IRBNet and I need to submit an amendment. What should I do?
It is important to not submit the changes with your initial submission.
If you need to amend an already approved study, you will first need to create a new project in IRB Novelution and input the latest version of your IRBNet project details into the respective IRB Novelution field. Use the IRBNet to IRB Novelution Comparison Guide as a reference. Be certain to include your IRBNet # in the Primary Information Section. This can be done by copying/ pasting from the latest version of your IRB approved application (Form 01) or protocol. Note this will also generate a new IRB number.
Please be certain to complete all applicable fields in IRB Novelution, as this will be what you will amend moving forward. Include documents such as consents, data collection tools, recruitment scripts, and other supplemental materials. Once you have done this, you may submit for review.
The IRB staff will provide you with an administrative review letter. Upon receipt of this letter, you may then proceed with your amendment, following the steps listed in the IRB Novelution tutorial videos/ how-to manual. Note that Amendment Requests (Form 03) are no longer required.
I need to submit a continuing review for my project. What should I do?
You may receive a notice from IRBNet that your project is due for continuing review. You will not be able to complete this task in IRBNet. If you need to submit for continuing review you will first need to create a new project in IRB Novelution and input the latest version of your IRBNet project details into the respective IRB Novelution field. Use the IRBNet to IRB Novelution Comparison Guide as a reference. Be certain to include your IRBNet # in the Primary Information Section. This can be done by copying/ pasting from the latest version of your IRB approved application (Form 01) or protocol. Note this will also generate a new IRB number.
Please be certain to complete all applicable fields in IRB Novelution, as this will be what you will use for all actions moving forward. Include documents such as consents, data collection tools, recruitment scripts, and other supplemental materials. Once you have done this, you may submit for review.
The IRB staff will provide you with an administrative review letter. Upon receipt of this letter, you may then proceed with your continuing review, following the steps listed in the IRB Novelution tutorial videos/ how-to manual. Note that Continuing Review Requests (Form 02) are no longer required.
I need to submit a progress report for my project. What should I do?
You may receive a notice from IRBNet that your project is due for a progress report. You will not be able to complete this task in IRBNet. If you need to submit for a progress report, you will first need to create a new project in IRB Novelution and input the latest version of your IRBNet project details into the respective IRB Novelution field. Use the IRBNet to IRB Novelution Comparison Guide as a reference. Be certain to include your IRBNet # in the Primary Information Section. This can be done by copying/ pasting from the latest version of your IRB approved application (Form 01) or protocol. Note this will also generate a new IRB number.
Please be certain to complete all applicable fields in IRB Novelution, as this will be what you will use for all actions moving forward. Include documents such as consents, data collection tools, recruitment scripts, and other supplemental materials. Once you have done this, you may submit for review.
The IRB staff will provide you with an administrative review letter. This will serve as your acknowledgement of progress. Note that Progress Reports for Minimal Risk Research (Form 02A) are no longer required.
My study is finished and I need to close it. What should I do?
If your project was only ever reviewed through IRBNet and you need to close it, complete Form 111 Study Closure Form. This form should be signed by the PI and submitted to researchintegrity@fau.edu. We will review and close the project in IRBNet. Upon receipt of your closure letter, make copies of all IRBNet documents for your reference. You will be required to maintain these documents for a minimum of three years after study closure.
If you have a IRB Novelution project that needs to be closed, follow the steps listed in the Novleution tutorial videos/ how-to manual for closing studies.
I received a Modifications Required letter in IRBNet? What do I do?
If you have already received a review from the Florida Atlantic IRB and were sent a “Modifications Required” letter, you should create a new project in Novleution incorporating changes requested by the IRB into this submission. Use the IRBNet to IRB Novelution Comparison Guide as a reference. Be certain to include your IRBNet # in the Primary Information Section. Complete all applicable fields in IRB Novelution, as this will be what you will use for all actions moving forward. Include documents such as consents, data collection tools, recruitment scripts, and other supplemental materials. Once you have done this, you may submit for review.
I need to submit a report for a serious adverse event or protocol violation. What should I do?
Send an email with details of the event to researchintegrity@fau.edu and we will advise you on next steps.
How do I know my college has signed / that the IRB Office has received my submission?
Log into your project and click the Requirements link in the Panel Shortcuts column on the left-hand side to view the workflow in the panel at the bottom.